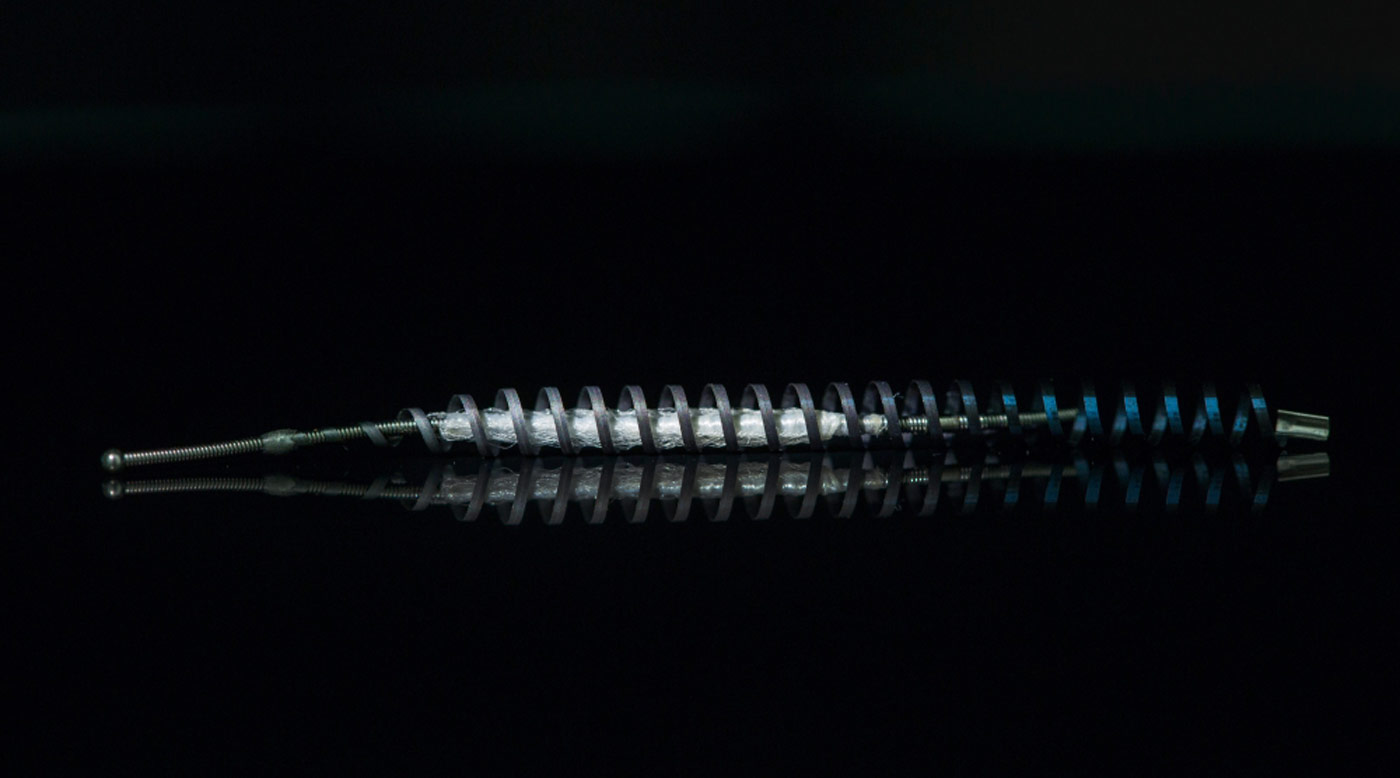
The German pharmaceutical giant Bayer has agreed to pay $1.6 billion to settle tens of thousands of United States claims related to its birth control device Essure.
Nearly 39,000 women across the U.S. had claims against Bayer after suffering health problems including severe pain, bleeding and organ damage after being implanted with the device, a contraceptive coil that is placed in the fallopian tubes. The settlement will resolve roughly 90% of these claims, Bayer said in a statement last week.
The agreement did not include an admission of wrongdoing or liability by Bayer, and plaintiffs who participate will be required to drop their claims.
“Bayer sympathizes with all women who have experienced adverse health conditions, regardless of the cause, but the company continues to stand by the science supporting the safety and efficacy of Essure,” Bayer said.
The settlement provides a coda to years of controversy that have surrounded Essure. Among the allegations made in the lawsuits was that Bayer and its predecessor, Conceptus, had failed to disclose information about patients who reported being harmed by the device. Conceptus developed Essure and was acquired by Bayer in 2013.
For years, Conceptus reported only a fraction of safety complaints by women about the device to the U.S. Food and Drug Administration, the International Consortium of Investigative Journalists reported in its 2018 Implant Files investigation. As a result, doctors, patients and regulators were left in the dark about the severity of the safety problems associated with the device.
Essure remained on the market as suspected injuries linked to the device surged from 25 in 2010 to 1,788 in 2014 and 12,564 in 2017. In 2018, the FDA ordered a sales restriction. Soon after, Bayer announced it was withdrawing the product from the U.S. market, the last country where it was still sold, a decision that it said it made for business reasons.
In previous statements to ICIJ, Bayer said that it followed FDA guidelines and was not legally required to report the earlier incidents to regulators.
Recently disclosed documents in California litigation against Essure include testimony by experts who alleged that Conceptus and Bayer had in fact violated FDA rules and engaged in systematic underreporting of injuries, ICIJ reported this month. An analysis by plaintiffs’ experts found that out of a sample of over 5,000 complaints filed over more than a decade, 24% of complaints should have been reported to the FDA but only 5.5% were actually reported.
Bayer disputes the claims and has dismissed the statistical analysis underpinning them as tainted by flawed methodology.
The $1.6 billion settlement will include the consolidated cases in California, according to Bayer, putting an end to the California litigation and its related disclosures.