What if I want to donate organs but not tissue?
Countries have widely varying laws surrounding organ and tissue donation — a few have no laws at all. In many countries, every citizen is considered a donor unless he chooses to opt out while he is still alive. In the United States, citizens must specifically consent. But each U.S. state has its own laws determining what that really means. Donate Life America offers a great resource for anyone seeking to register as an organ or tissue donor — or anyone who wants to define what the gift should be.
Companies can make a profit from my body. But aren’t I saving lives?
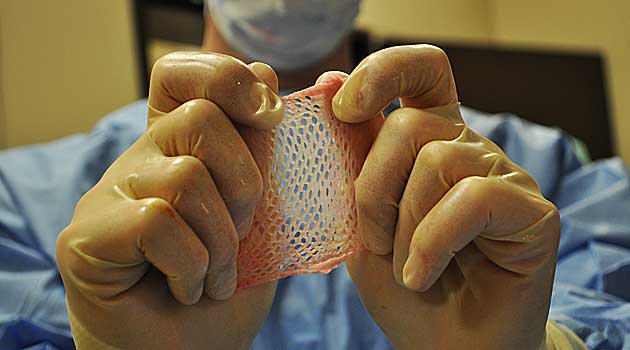
Tissues improve the quality of peoples’ lives. And in some cases, yes, they even save lives. Heart valves are in short supply, for instance. Skin is used as a cover for severe burns — it works better than any synthetic covering when those burns cover a majority of the body — and to help slow-healing diabetic foot ulcers. Corneas can be used to help someone see again. Bone can be milled into implants that correct spinal deformities.
But tissue is also used in a number of cosmetic surgeries such as fattening lips or shaping a new nose. Skin can be used as an injectable to fill fine lines and wrinkles.
Should I be concerned about safety?
We can’t say for sure, because infections are not always tracked effectively. And it’s hard to determine in most cases whether an infection resulted from a tissue graft or from, say, the scalpel the surgeon used in the operation. But the industry estimates that infections are rare. And we haven’t found information that disagrees with that.
Risk of infection depends on the type of tissue you receive. Most bone used in dental and other general surgery is sterilized. But some tissue can’t be industrially sterilized, such as “fresh” bone and tendon grafts, corneas, veins or heart valves. In those cases, the public largely relies on donor screening to avoid transmission of disease or bacteria.
Can I find out about the tissue bank in my area? Whether it’s been inspected, reported any adverse events or accidents, or been involved in any recalls?
The FDA keeps a registry of tissue banks that do business in the U.S. That includes everything from sperm banks to laboratories that run tests on stem cells. You can check if the bank has been inspected, but you would need to file a records request if you wanted to read the concerns FDA auditors might have documented.
It’s harder to find out if a bank has reported adverse events or accidents. Many adverse events are filed voluntarily, so the FDA won’t release those. But some tissues are regulated as a medical devise rather than a biologic. Reports of those adverse events can be found in the FDA’s Manufacturer and User Facility Device Experience.
Reports of errors and accidents in a tissue bank’s manufacturing process are not available on line. But they are on public record, so you can file a records request.
Finally, recalls are reported either as biologics or as medical devices and are available at the FDA website.
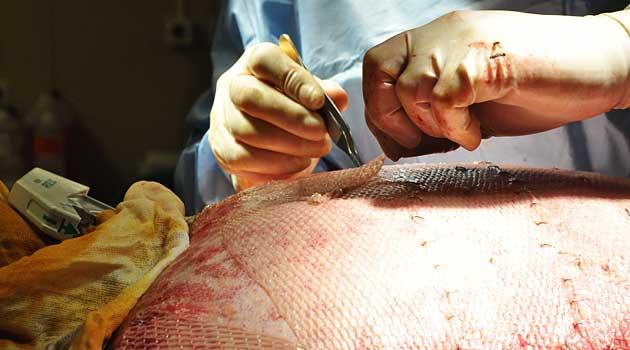
I had orthopedic surgery. Did I receive tissue from a cadaver?
Maybe. Doctors are not required to inform patients when they use human tissue during an operation. So ask your doctor what product was used. You can probably find it at the FDA.
How can I track the source of the human tissue I was implanted with?
We don’t know.
Do you have any other questions about the use of human tissue as medical devices or the global trade of this material? Get in touch via contact@icij.org.